 | |||||||||||||||
go to
ARCHIVE
for topics on
all the days.
ARCHIVE
2010
apr days
• color
concept &
theory widgets and apps
mar days
• red:
a portrait of a artist rothko
feb days
• talking
heads as figure/ground
jan days
• tanja's
black light dance party
dec days
• tootsie roll pop wrappers colors & flavors
nov days
• stephen vitiello's four color sound
oct days
• atmospheric perspective
sept days
• a rainbow
of antioxidants
colors
aug days
• floor stain colorants
jul days
• minimal colors
jun days
• wildflowers cataloged by color
may days
• tennis court colors
apr days
• morandi's neutral colors
mar days
• grid colorists
feb days
• black as
film noir
jan days
• flood of toxic minerals used in paints
ARCHIVE
2008
dec days
• comple-mentary
colors
nov days
• kettle korn
packaging color change
oct days
• green fluorescent protein
sept days
• red palms - not green
aug days
• blue tunes
jul days
• “blue” - textile museum
jun days
• “fiesta- ware”
colorants
may days
• “blue alchemy” hive gallery
apr days
• “sennelier” selecting
watercolours for travel


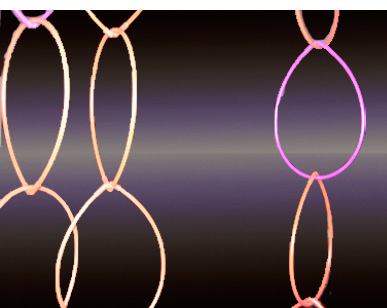
black light and glow rings
Using black light, the gin
and tonic glows in the dark.
Photo taken of Tanja by Jeff in January, 2009
The colors we see are determined by the wave-length of light energy. Unlike some insects, humans can only view the spectrum from red to violet. However, other invisible “colors” exist above and below this spectrum.
The “color” above red is called infra-red and the color below violet is called ultra-violet. Ultraviolet light will cause fluorescent or phosphorescent pigments to fluoresce, emitting visible light. – Glow Inc. Link
The black light experiment happens again at Tanja's annual party with another gin and tonic in January 2010.
Party glow rings.
The glow light is made of
two parts which when mixed together create the chemical reaction which makes them glow.
4 g sodium carbonate
0.2 g luminol
0.5 g ammonium carbonate
0.4 g copper sulfate pentahydrate
approx. 1 litre of distilled water.
50 ml of 3% hydrogen peroxide.
Glow sticks work by a chemical reaction called “chemiluminescence” which causes light
to be generated. (That's a trivia question for some party games!) By breaking up the word "chemiluminescence" into 2 parts, chemi and luminescence we can better under-stand. Luminescence is emission of light not caused by a rise in temperature (the object emitting light stays cold). The light is released by atoms returning from "excited" (charged) state to normal "ground" state. It means, an object which glows, first must absorb energy to get into "excited state." That energy will be released (in form of light) by the object returning to the ground state of energy.
– China Bessen Glow Technology Ltd. Link
click here to read
more on this topic
October Days, 2008
green fluorescent protein